Dithiafulvene derivatized donor-acceptor norbornadienes with redshifted absorption
M. Mansø,
M. D. Kilde,
S. K. Singh,
P. Erhart,
K. Moth-Poulsen,
and
M. Brøndsted Nielsen
Physical Chemistry Chemical Physics 21, 3092
(2019)
doi: 10.1039/C8CP07744D
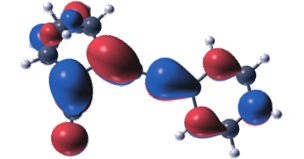
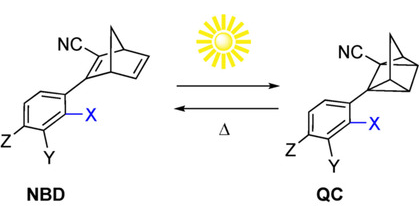
Photoisomerization of norbornadiene (N) to its metastable isomer quadricyclane (Q) has attracted interest as a strategy for harvesting and storing solar energy. For this strategy to mature the absorption maximum of N has to be moved from the UV to the visible region. Here we show that functionalization of the system with dithiafulvene (DTF) electron donors causes remarkable redshifts of various N derivatives. Thus, some derivatives were found to absorb light with an absorption onset up to 556 nm. The incorporation of DTF units comes, however, with a drawback with regard to achieving reversible N-to-Q and Q-to-N isomerizations. For some derivatives, the photoisomerization was completely quenched. The compounds were subjected to a computational study to shed light on the underlying reason for this reluctance to undergo photoisomerization. The computational study revealed that in these systems, the first excited state (S1) is positioned close to or lower than the transition state for photoconversion, effectively blocking a possible conversion to Q, thus revealing a practical challenge for the future design of N–Q energy storage systems with an improved solar spectrum match.